Continuous process for coating liposomial vectors with polymer
The “ Bioadhesive liposomes” project consists of the production of nano-liposomal vectors coated with bioadhesive polymers for nutraceutical applications. Since 2019 Eng4Life owns the patent “Continuous process for coating liposomial vectors with polymer”.
The patent can be consulted at the following link.
The production technique of Eng4Life for liposomes is SMF – Simil Microfluidic that can be used to polymeric coating of the nanoliposomes vectors to produce shell-core nanostructures.
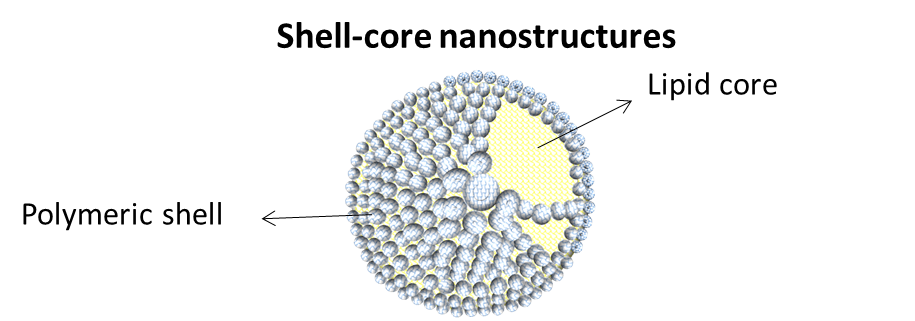
In the nutraceutical field, shell-core structures can be used for the dosing of micronutrients, bringing three main advantages:
1)
|
Increase in the residence time of the molecule on the site of action
|
2)
|
Improvement of the bioavailability of the encapsulated molecule
|
3)
|
Increased paracellular permeability
|
There are several techniques that can be used for the superficial polymeric coating of nanoliposomes, such as the dripping method (Drop-wise); Reverse-Phase Evaporation (REV); Improved Supercritical Reverse-Phase Evaporation (ISCRPE); etc …
These techniques present problems:

The SMF technique used by Eng4Life is a rapid and continuous process that guarantees:
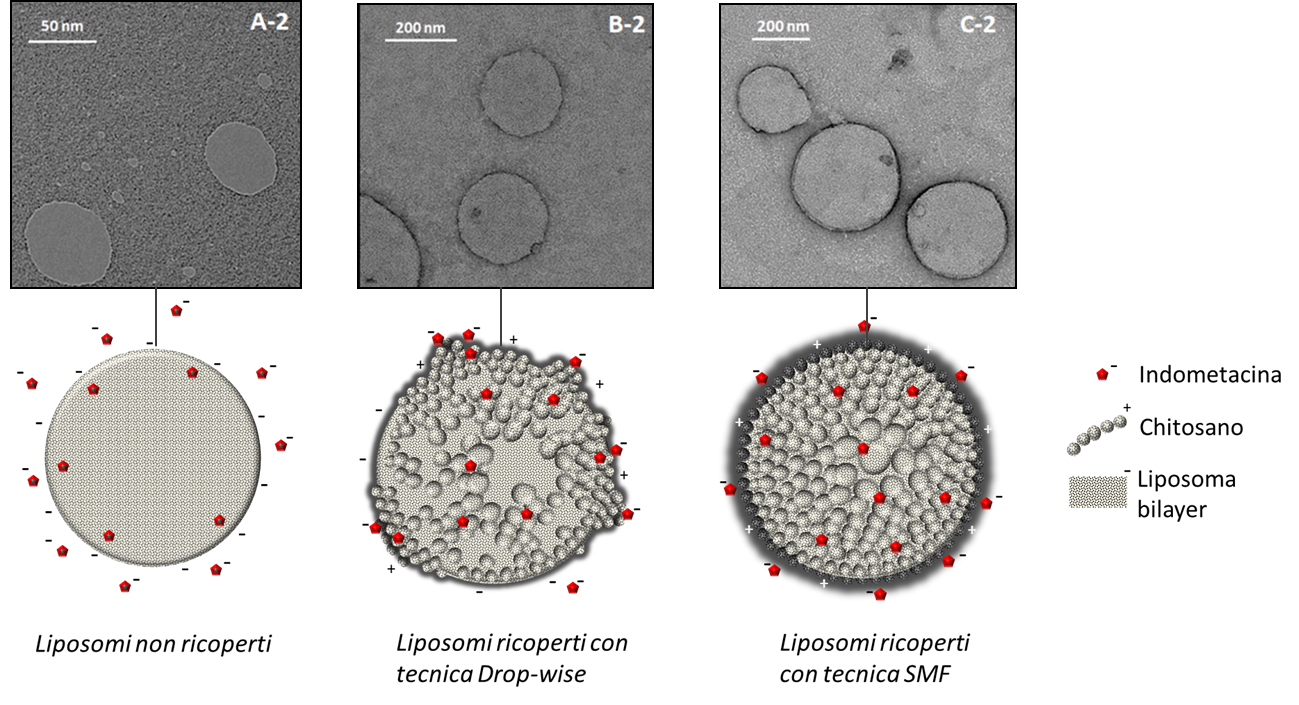
To demonstrate its effectiveness, the SMF method was compared with the drop-wise method, as shown in the following image, the coating made with the SMF method returns shell-core particles with a thick, smooth and homogeneous polymeric surface (C2 ) those obtained with the drop-wise method show a very thin, rough and irregular coating (B2).
Liposomes are vectors that can be used for the controlled release of different types of active molecules and, thanks to their unique characteristics, are widely used in various fields of application such as pharmaceutical, medical, nutraceutical, cosmoceutic.
In the food sector, liposomes have great potential as “container” materials to preserve many bioactive ingredients, including compounds that are poorly soluble in water, vitamins, proteins, peptides, polyphenols etc.
The team of researchers of Eng4Life is engaged in the development of the processes of production of encapsulating liposomes active molecules, obtained through the described patented technique.
Here are some of the publications on the subject made by Eng4life members.
2021
Bochicchio, Sabrina; Dalmoro, Annalisa; Lamberti, Gaetano; Barba, Anna Angela
Nanoliposomi in cosmetica e cosmeceutica Journal Article
In: ICF – Rivista dell’industria chimica e farmaceutica, vol. Febbraio/Marzo 2021, no. 1, pp. 66-71, 2021.
@article{Bochicchio2021,
title = {Nanoliposomi in cosmetica e cosmeceutica},
author = {Sabrina Bochicchio and Annalisa Dalmoro and Gaetano Lamberti and Anna Angela Barba},
url = {https://interprogettied.com/icf-rivista-dellindustria-chimica-e-farmaceutica-n1-febbraio-marzo-2021/},
year = {2021},
date = {2021-03-26},
journal = {ICF – Rivista dell’industria chimica e farmaceutica},
volume = {Febbraio/Marzo 2021},
number = {1},
pages = {66-71},
abstract = {L’uso di nanotecnologie per potenziare le performance dei prodotti cosmetici e cosmeceutici ha consentito una rapida crescita di questo settore industriale. Registrano un’ampia richiesta di mercato i nanocosmeceutici per la cura della pelle, dei capelli, delle unghie e delle labbra, oltre a quelli per contrastare rughe, fotoinvecchiamento, iperpigmentazione, forfora e danni ai capelli.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
L’uso di nanotecnologie per potenziare le performance dei prodotti cosmetici e cosmeceutici ha consentito una rapida crescita di questo settore industriale. Registrano un’ampia richiesta di mercato i nanocosmeceutici per la cura della pelle, dei capelli, delle unghie e delle labbra, oltre a quelli per contrastare rughe, fotoinvecchiamento, iperpigmentazione, forfora e danni ai capelli.
2019
Barba, Anna Angela; Bochicchio, Sabrina; Bertoncin, Paolo; Lamberti, Gaetano; Dalmoro, Annalisa
Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches Journal Article
In: Coatings, vol. 9, no. 8, pp. 491, 2019.
@article{Barba2019,
title = {Coating of Nanolipid Structures by a Novel Simil-Microfluidic Technique: Experimental and Theoretical Approaches },
author = {Anna Angela Barba and Sabrina Bochicchio and Paolo Bertoncin and Gaetano Lamberti and Annalisa Dalmoro},
url = {https://www.mdpi.com/2079-6412/9/8/491/htm},
doi = {10.3390/coatings9080491},
year = {2019},
date = {2019-08-02},
journal = {Coatings},
volume = {9},
number = {8},
pages = {491},
abstract = {Nanolipid vesicular structures are ideal candidates for the controlled release of various ingredients, from vitamins for nutraceutical purposes to chemoterapic drugs. To improve their stability, permeability, and some specific surface properties, such as mucoadhesiveness, these structures can require a process of surface engineering. The interaction of lipid vesicles with oppositely charged polyelectrolytes seems to be an interesting solution, especially when the negatively charged liposomes are complexed with the cationic chitosan. In this work, a novel simil-microfluidic technique was used to produce both chitosan-coated vesicles and a vegan alternative composed of cholesterol-free liposomes coated by Guar Hydroxypropyltrimonium Chloride (Guar-HC). The combination between the experimental approach, based on experimental observations in terms of Z-potential, and size evolutions, and the theoretical approach, based on concepts of saturation, was the methodology applied to define the best polycation concentration to fairly cover (vegan or not) liposomes without aggregation. The smart production of coated nanolipid structures was confirmed by characterizations of morphology, mucoadhesiveness, and stability.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Nanolipid vesicular structures are ideal candidates for the controlled release of various ingredients, from vitamins for nutraceutical purposes to chemoterapic drugs. To improve their stability, permeability, and some specific surface properties, such as mucoadhesiveness, these structures can require a process of surface engineering. The interaction of lipid vesicles with oppositely charged polyelectrolytes seems to be an interesting solution, especially when the negatively charged liposomes are complexed with the cationic chitosan. In this work, a novel simil-microfluidic technique was used to produce both chitosan-coated vesicles and a vegan alternative composed of cholesterol-free liposomes coated by Guar Hydroxypropyltrimonium Chloride (Guar-HC). The combination between the experimental approach, based on experimental observations in terms of Z-potential, and size evolutions, and the theoretical approach, based on concepts of saturation, was the methodology applied to define the best polycation concentration to fairly cover (vegan or not) liposomes without aggregation. The smart production of coated nanolipid structures was confirmed by characterizations of morphology, mucoadhesiveness, and stability.
Dalmoro, Annalisa; Bochicchio, Sabrina; Lamberti, Gaetano; Bertoncin, Paolo; Janssens, Barbara; Barba, Anna Angela
Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology Journal Article
In: RSC Advances, vol. 9, pp. 19800-19812, 2019.
@article{Dalmoro2019,
title = {Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology },
author = {Annalisa Dalmoro and Sabrina Bochicchio and Gaetano Lamberti and Paolo Bertoncin and Barbara Janssens and Anna Angela Barba},
url = {https://pubs.rsc.org/en/content/articlelanding/2019/ra/c9ra03022k},
doi = {10.1039/C9RA03022K},
year = {2019},
date = {2019-06-25},
journal = {RSC Advances},
volume = {9},
pages = {19800-19812},
abstract = {Micronutrients administration by fortification of staple and complementary foods is a followed strategy to fight malnutrition and micronutrient deficiencies and related pathologies. There is a great industrial interest in preparation of formulations for joint administration of vitamin D3 and vitamin K2 for providing bone support, promoting heart health and helping boost immunity. To respond to this topic, in this work, uncoated nanoliposomes loaded with vitamin D3 and K2 were successfully prepared, by using a novel, high-yield and semi continuous technique based on simil-microfluidic principles. By the same technique, to promote and to enhance mucoadhesiveness and stability of the produced liposomal structures, chitosan was tested as covering material. By this way polymer–lipid hybrid nanoparticles, encapsulating vitamin D3 and vitamin K2, with improved features in terms of stability, loading and mucoadhesiveness were produced for potential nutraceutical and pharmaceutical applications.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Micronutrients administration by fortification of staple and complementary foods is a followed strategy to fight malnutrition and micronutrient deficiencies and related pathologies. There is a great industrial interest in preparation of formulations for joint administration of vitamin D3 and vitamin K2 for providing bone support, promoting heart health and helping boost immunity. To respond to this topic, in this work, uncoated nanoliposomes loaded with vitamin D3 and K2 were successfully prepared, by using a novel, high-yield and semi continuous technique based on simil-microfluidic principles. By the same technique, to promote and to enhance mucoadhesiveness and stability of the produced liposomal structures, chitosan was tested as covering material. By this way polymer–lipid hybrid nanoparticles, encapsulating vitamin D3 and vitamin K2, with improved features in terms of stability, loading and mucoadhesiveness were produced for potential nutraceutical and pharmaceutical applications.
2018
Bochicchio, Sabrina; Dalmoro, Annalisa; Bertoncin, Paolo; Lamberti, Gaetano; Moustafine, Rouslan I.; Barba, Anna Angela
Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: conventional and next-generation approaches Journal Article
In: RSC Advances, vol. 8, pp. 34614–34624, 2018.
@article{Bochicchio2018,
title = {Design and production of hybrid nanoparticles with polymeric-lipid shell–core structures: conventional and next-generation approaches },
author = {Sabrina Bochicchio and Annalisa Dalmoro and Paolo Bertoncin and Gaetano Lamberti and Rouslan I. Moustafine and Anna Angela Barba },
url = {https://pubs.rsc.org/en/Content/ArticleLanding/2018/RA/C8RA07069E#!divAbstract},
doi = {10.1039/c8ra07069e},
year = {2018},
date = {2018-09-27},
journal = {RSC Advances},
volume = {8},
pages = {34614–34624},
abstract = {Liposomes constitute a class of prominent drug delivery systems due their cell-mimetic behaviour. Despite
their high biocompatibility, biodegradability and low intrinsic toxicity, their poor stability in biological fluids
as well as in stock conditions (high tendency to degrade or aggregate) have led to new approaches for
liposome stabilization (e.g., surface covering with polymers). Here, liposomes were enwrapped by the
natural biocompatible polymer chitosan to achieve stable shell–core nanostructures. Covered
nanoliposomes were produced using an innovative continuous method based on microfluidic principles.
The produced hybrid polymeric-lipid nanoparticles were characterized in terms of structural properties,
size and stability. Moreover, phenomenological aspects in formation of nanoliposomal vesicles and
chitosan layering, product quality (structure, size) and manufacturing yield related to this novel method
were compared with those of the conventional dropwise method and the obtained products. The
proposed simil-microfluidic method led to the production of stable and completely chitosan-covered
liposomes with a shell–core nanostructure that avoided the disadvantages inherent in the conventional
method (which are time-consuming and/or require bulky and more expensive equipment).},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Liposomes constitute a class of prominent drug delivery systems due their cell-mimetic behaviour. Despite
their high biocompatibility, biodegradability and low intrinsic toxicity, their poor stability in biological fluids
as well as in stock conditions (high tendency to degrade or aggregate) have led to new approaches for
liposome stabilization (e.g., surface covering with polymers). Here, liposomes were enwrapped by the
natural biocompatible polymer chitosan to achieve stable shell–core nanostructures. Covered
nanoliposomes were produced using an innovative continuous method based on microfluidic principles.
The produced hybrid polymeric-lipid nanoparticles were characterized in terms of structural properties,
size and stability. Moreover, phenomenological aspects in formation of nanoliposomal vesicles and
chitosan layering, product quality (structure, size) and manufacturing yield related to this novel method
were compared with those of the conventional dropwise method and the obtained products. The
proposed simil-microfluidic method led to the production of stable and completely chitosan-covered
liposomes with a shell–core nanostructure that avoided the disadvantages inherent in the conventional
method (which are time-consuming and/or require bulky and more expensive equipment).
Dalmoro, Annalisa; Bochicchio, Sabrina; Nasibullin, Shamil F.; Bertoncin, Paolo; Lamberti, Gaetano; Barba, Anna Angela; Moustafine, Rouslan I.
Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems Journal Article
In: European Journal of Pharmaceutical Sciences, vol. 121, pp. 16-28, 2018.
@article{Dalmoro2018,
title = {Polymer-lipid hybrid nanoparticles as enhanced indomethacin delivery systems},
author = {Annalisa Dalmoro and Sabrina Bochicchio and Shamil F. Nasibullin and Paolo Bertoncin and Gaetano Lamberti and Anna Angela Barba and Rouslan I. Moustafine},
url = {https://www.sciencedirect.com/science/article/pii/S0928098718302331},
doi = {10.1016/j.ejps.2018.05.014},
year = {2018},
date = {2018-08-30},
journal = {European Journal of Pharmaceutical Sciences},
volume = {121},
pages = {16-28},
abstract = {Non-steroidal anti-inflammatory drugs (NSAIDs), i.e. indomethacin used for rheumatoid arthritis and non-rheumatoid inflammatory diseases, are known for their injurious actions on the gastrointestinal (GI) tract. Mucosal damage can be avoided by using nanoscale systems composed by a combination of liposomes and biodegradable natural polymer, i.e. chitosan, for enhancing drug activity.
Aim of this study was to prepare chitosan-lipid hybrid delivery systems for indomethacin dosage through a novel continuous method based on microfluidic principles. The drop-wise conventional method was also applied in order to investigate the effect of the two polymeric coverage processes on the nanostructures features and their interactions with indomethacin. Thermal-physical properties, mucoadhesiveness, drug entrapment efficiency, in vitro release behavior in simulated GI fluids and stability in stocking conditions were assayed and compared, respectively, for the uncoated and chitosan-coated nanoliposomes prepared by the two introduced methods.
The prepared chitosan-lipid hybrid structures, with nanometric size, have shown high indomethacin loading (about 10%) and drug encapsulation efficiency up to 99%. TEM investigation has highlighted that the developed novel simil-microfluidic method is able to put a polymeric layer, surrounding indomethacin loaded nanoliposomes, thicker and smoother than that achievable by the drop-wise method, improving their storage stability. Finally, double pH tests have confirmed that the chitosan-lipid hybrid nanostructures have a gastro retentive behavior in simulated gastric and intestinal fluids thus can be used as delivery systems for the oral-controlled release of indomethacin.
Based on the present results, the simil-microfluidic method, working with large volumes, in a rapid manner, without the use of drastic conditions and with a precise control over the covering process, seems to be the most promising method for the production of suitable indomethacin delivery system, with a great potential in industrial manufacturing.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Non-steroidal anti-inflammatory drugs (NSAIDs), i.e. indomethacin used for rheumatoid arthritis and non-rheumatoid inflammatory diseases, are known for their injurious actions on the gastrointestinal (GI) tract. Mucosal damage can be avoided by using nanoscale systems composed by a combination of liposomes and biodegradable natural polymer, i.e. chitosan, for enhancing drug activity.
Aim of this study was to prepare chitosan-lipid hybrid delivery systems for indomethacin dosage through a novel continuous method based on microfluidic principles. The drop-wise conventional method was also applied in order to investigate the effect of the two polymeric coverage processes on the nanostructures features and their interactions with indomethacin. Thermal-physical properties, mucoadhesiveness, drug entrapment efficiency, in vitro release behavior in simulated GI fluids and stability in stocking conditions were assayed and compared, respectively, for the uncoated and chitosan-coated nanoliposomes prepared by the two introduced methods.
The prepared chitosan-lipid hybrid structures, with nanometric size, have shown high indomethacin loading (about 10%) and drug encapsulation efficiency up to 99%. TEM investigation has highlighted that the developed novel simil-microfluidic method is able to put a polymeric layer, surrounding indomethacin loaded nanoliposomes, thicker and smoother than that achievable by the drop-wise method, improving their storage stability. Finally, double pH tests have confirmed that the chitosan-lipid hybrid nanostructures have a gastro retentive behavior in simulated gastric and intestinal fluids thus can be used as delivery systems for the oral-controlled release of indomethacin.
Based on the present results, the simil-microfluidic method, working with large volumes, in a rapid manner, without the use of drastic conditions and with a precise control over the covering process, seems to be the most promising method for the production of suitable indomethacin delivery system, with a great potential in industrial manufacturing.





